Kenya has once again stepped into the global spotlight in the fight against HIV.
In a groundbreaking development, the country has been selected as one of only nine nations worldwide to introduce Lenacapavir, a revolutionary HIV prevention drug that requires just two injections per year.
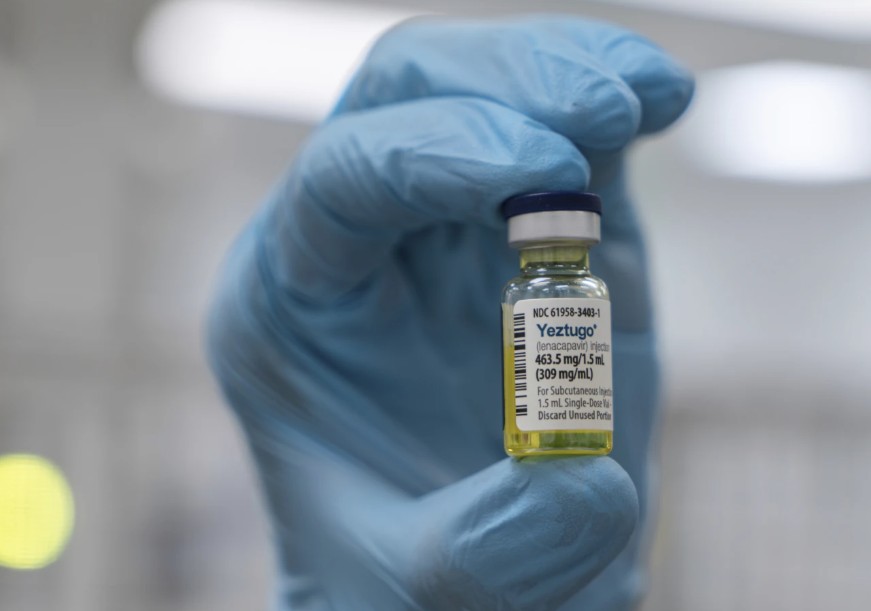
Branded as Yeztugo, Lenacapavir was recently approved by the World Health Organization (WHO) following promising clinical trials that showed complete protection against HIV infection, particularly among women and adolescent girls, the population most at risk in sub-Saharan Africa.
The Ministry of Health, through its National AIDS and STI Control Programme (NASCOP), has announced that the new drug will be available in Kenya by January 2026.
What sets Lenacapavir apart from existing prevention methods is its ultra-long-acting formula.
Unlike daily oral pre-exposure prophylaxis (PrEP) that requires consistent, daily adherence, Lenacapavir is administered only once every six months. This could significantly improve adherence and protection rates.
In clinical trials, the injectable drug showed remarkable efficacy, with zero infections among the women who received it, leading experts to describe it as a “potential game changer.”
“This is a major milestone in our ongoing efforts to end HIV transmission in Kenya,” said a NASCOP spokesperson. “Yeztugo offers a powerful new option, especially for young women, who often face challenges accessing or adhering to daily oral PrEP.”